Women between the ages of 40 and 44 who have tried and failed to conceive may benefit from the addition of a growth hormone to traditional IVF therapy, according to a leading fertility expert, Dr David Knight.
And to help prove the thesis, Dr Knight’s IVF clinic in Sydney is seeking “Australia’s most reproductively challenged women” to take part in a groundbreaking fertility trial using growth hormone - the first of its kind to be conducted in Australia.
The trial will be led Dr Knight who is the Medical Director at Demeter Fertility. It will measure the effect of adding a recombinant Human Growth Hormone (rHGH) to traditional IVF therapy in women aged 40-44 years over a 10-week period.
“Fertility rates decline significantly with age and by the time a woman reaches
40 her chances of conceiving naturally in one cycle has fallen to about 5%,” Dr Knight said.
“Even with IVF therapy a woman in her early 40s still has less than a 10% chance of falling pregnant each cycle.”
According to Dr Knight, one in four IVF treatments performed in Australia and New Zealand involve women over the age of 40.
A previous overseas study has shown that the addition of a growth hormone to traditional IVF can result in a five-fold increase in successful pregnancies and a lower rate of miscarriage amongst older women.
“We believe there is potential for pregnancy and live birth rates to be greatly improved by adding the growth hormone to current IVF therapy,” said Dr Knight.
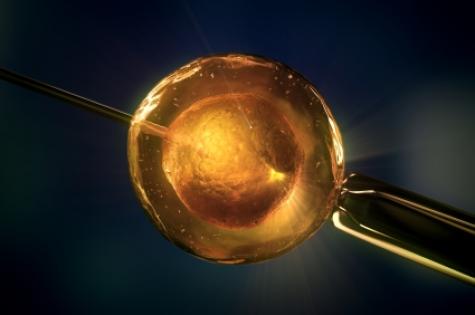
In vitro fertilisation involves the fertilisation of several eggs outside of the body in the hope that one will become a viable embryo.
“As a woman ages, the number of suitable eggs available for this therapy diminishes and that’s why this new method is so important. We believe that by using a growth hormone we can enhance the strength and growth rate of a single egg and potentially improve the chance of fertilisation,” said Dr Knight.
Demeter Fertility is seeking 120 couples prepared to take part in the 10-week trial. All participants will be required to undergo a preliminary health check and female participants will need to be between 40 and 44 years of age.
Demeter Fertility has received Ethics Committee approval according to the National Statement and the trial will be conducted in accordance with Good Clinical Practice Guidelines. Once completed, the study results will be submitted to the Therapeutic Goods Administration.
* * *
For more information about the trial, visit DemeterFertility.com.


















__small.png)










